G-PUR ® is a pioneer in dietary supplement innovation, specializing in high-quality, effective products like G-PUR®. Our mission is to enhance consumer health by providing innovative solutions that prevent the absorption of harmful substances, uphold quality, and safety standards.
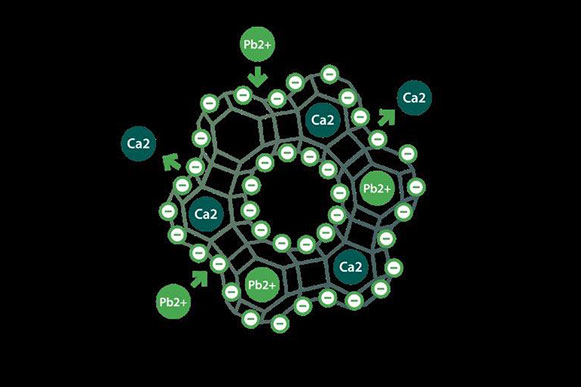
Discover G-PUR ®, a zeolite mineral a groundbreaking dietary supplement, brought to you exclusively by G-Science, Inc. Our product is distinguished by its patented purification process and New Dietary Ingredient (NDI) status, emphasizing its uniqueness and superiority in the market. G-Science, Inc. is the only company to file an NDIN for a purified zeolite mineral.
Our Commitment:
- Delivering innovative health solutions.
- Upholding stringent quality and safety standards.
- Enhancing consumer well-being through groundbreaking products.

Exclusive Distribution:
- G-Science is proud to be the sole distributor, emphasizing the provision of bulk quantities and the patented purification process of G-PUR ®.
Unique Differentiator:
- The patented purification and NDI status underscore G-PUR ® as a premium choice in the dietary supplement market, focusing on bulk sales and qualifying as a new ingredient in several supplement brands sold through practitioners uniqueness.

Recognition:
G-PUR ® has received acknowledgment from the FDA, affirming its safety and efficacy with a “No Objection” letter.
Our History
In cooperation with external, well-respected Academic Partners & Universities, research studies for other CLN applications intensified and are currently ongoing.