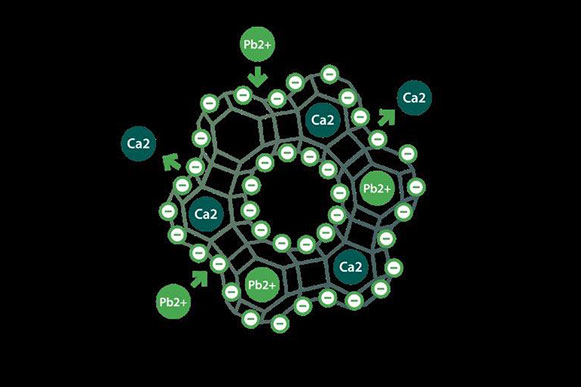
G-SCIENCE™ is the exclusive distributor of G-PUR® purified clinoptilolite. We aim at providing a broad range of dietary supplements (with a strong focus on primary, secondary and tertiary prevention). Our company covers a broad range of know-how and expertise from geology to biology, from analytical chemistry to clinical development, supported by well-respected universities and their excellent scientists. Our patented manufacturing process, secured by a certified quality management system, guarantees a one-of-a-kind and safe version of Clinoptilolite, which is further processed to introduce modifications that allow to target specific applications. Patients benefit from effective medicines with a low propensity to cause side effects. Our aim is to become the leading company in the field of Clinoptilolites/Zeolites with our culture of striving for excellence.

About
Our Mission
Commitment
The success of G-SCIENCE comes from our relentless desire to achieve the highest quality standards. We certainly pride ourselves on growth and innovation, and it is our dedication to ethical behavior, continuous quality improvement, open communication, trusted leadership and empowering teamwork that truly distinguishes G-SCIENCE. These characteristics inspire confidence in the people we serve across the world and reflect a philosophy present in everything we do. This commitment extends to patients and customers, our employees, our global communities and our environment.

Leadership
G-SCIENCE leads the industry by exceeding expectations. The status quo is never enough at G-SCIENCE. Each day we ask ourselves how we can improve on what we do, how we do it, and how we can lead the industry in new ways. So, in every department, at every location, our people strive to deliver high quality products and services in the most innovative ways. For instance, we know how important our pharmacy partners are to consumers and healthcare professionals, so we put significant effort into working closely with them in all they do.

Who We Are
G-SCIENCE, Inc is the exclusive distributor of G-PUR purified clinoptilolite.
G-SCIENCE partners with consumers, physicians and pharmacists to deliver the best therapies and solutions to improve lives. Our approach enables us to constantly adapt to a never-changing healthcare industry, so as needs change and grow, so do we. From education and leadership to advocacy, this personal passion for our customers’ well-being is at the heart of everything G-SCIENCE does.

Capabilities
Our products are inspired by the people we serve consumers, physicians and pharmacists rely on G-SCIENCE to produce only the finest therapies and healthcare solutions. We subject our products to a full regimen of testing and quality control. We begin the process with data-driven research and product development. Then our medical, regulatory and legal teams work diligently to thoroughly educate patients, caregivers and healthcare professionals about the effectiveness and safety of each product. Our quality assurance team ensures safety and efficacy of our products and strives to meet the regulatory standards put forth by the FDA. The result is a pure, safe and effective product every time.
Our History
In cooperation with external, well-respected Academic Partners & Universities, research studies for other CLN applications intensified and are currently ongoing.