G-PUR® is a leader in dietary supplement innovation, offering high-quality, effective products like G-PUR®, designed to enhance health and wellness. Through our exclusive partnership with GovX.com, we are proud to extend the benefits of G-PUR® to active and former military, first responders, and government employees. Our mission is to improve consumer health by developing innovative solutions that prevent the absorption of harmful substances, while maintaining the highest standards of quality and safety.
Serving High Quality Health Solutions:
The partnership between G-PUR® and GovX.com allows us to provide these benefits to a broader audience, particularly those who serve or have served. By offering G-PUR® on GovX.com, we ensure that military personnel, first responders, and government employees have access to high-quality health solutions that fit their unique needs. This partnership underscores our dedication to making our innovative products accessible to those who protect and serve.
Unique Differentiator:
- The patented purification and NDI status underscore G-PUR ® as a premium choice in the dietary supplement market, focusing on bulk sales and qualifying as a new ingredient in several supplement brands sold through practitioners uniqueness.

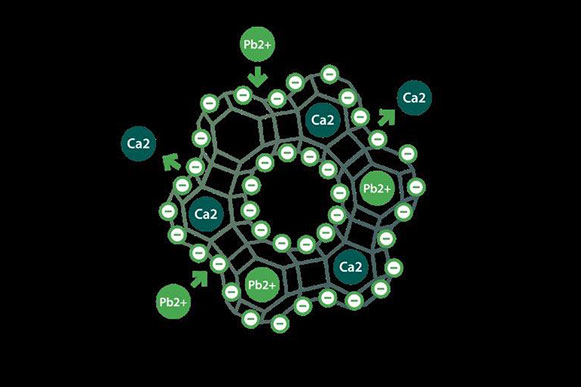
Discover G-PUR ®, a zeolite mineral a groundbreaking dietary supplement, brought to you exclusively by G-Science, Inc. Our product is distinguished by its patented purification process and New Dietary Ingredient (NDI) status, emphasizing its uniqueness and superiority in the market. G-Science, Inc. is the only company to file an NDIN for a purified zeolite mineral.
Our Commitment:
- Delivering innovative health solutions.
- Upholding stringent quality and safety standards.
- Enhancing consumer well-being through groundbreaking products.

Recognition:
G-PUR ® has received acknowledgment from the FDA, affirming its safety and efficacy with a “No Objection” letter.
Our History
In cooperation with external, well-respected Academic Partners & Universities, research studies for other CLN applications intensified and are currently ongoing.